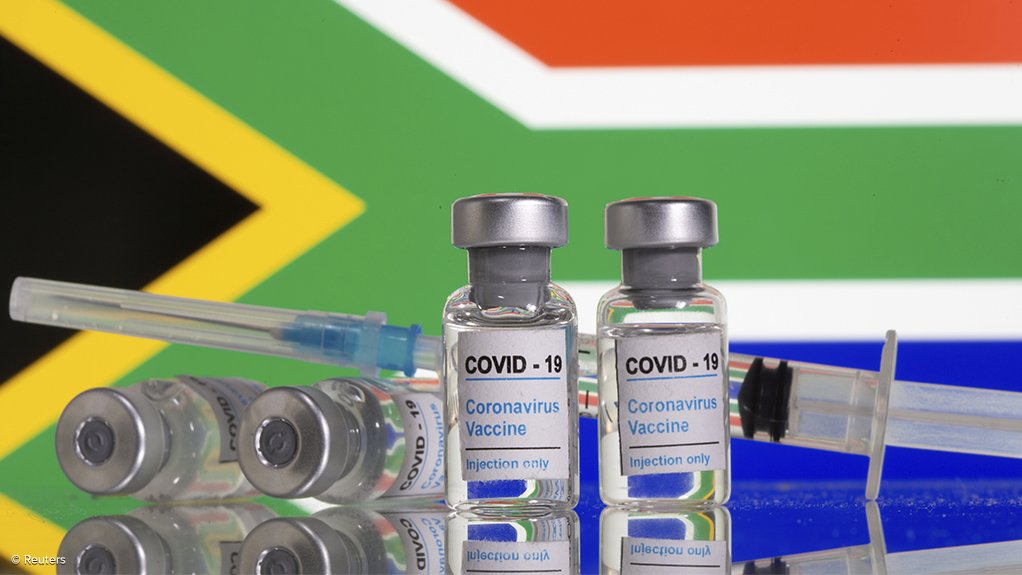
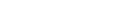The South African Health Products Regulatory Authority (SAHPRA) stressed on Monday that it could not approve any vaccines if an application is not submitted to the authority, as South Africa trails in its roll-out of Covid-19 vaccines.
However, the SAHPRA revealed that applications had been received for the Sputnik V and Sinovac’s Coronavac vaccines and that the evaluation of the Coronavac application is at an advanced stage.
The Sputnik V application is a rolling review, which the SAHPRA says is a mechanism that facilitates the submission of data as it becomes available.
“Whilst reviews can commence earlier with a rolling submission, it is important to note that some very important efficacy, quality and safety information is sometimes outstanding and would require review for consideration of such products for public use. Therefore, pharmaceutical companies can submit applications indicating a plan of when they will be submitting their data, i.e. outline when the next rolling submission sequence is available for review,” the authority explains.
SAHPRA has not made a decision on the Sinopharm and Moderna vaccines, which have a World Health Organization (WHO) Emergency Use Listing, as there have not been any applications for these vaccines in South Africa, the authority said.
SAHPRA has so far approved the Pfizer and the Johnson & Johnson vaccines for use in the country.
SAHPRA CEO Dr Boitumelo Semete-Makokotlela said the regulatory body would not be pressured to allow the public access to any product that had not met the necessary regulatory requirements.
The SAHPRA is concerned about the prevalence of other Covid-19 variants in South Africa, and is urging pharmaceutical companies to provide information on studies supporting efficacy with their vaccine applications.
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here